What Is the Formula Charles Law
As the temperature increases the volume of the gas also increases. What is the formula for Charles Law.

Charles Law Chemistry Lessons Chemistry Charles Law
This law applies to ideal gases held at a constant pressu madmax232004 madmax232004 04172020 Mathematics Middle School answered What is.

. This law was then used later on to determine the volume or temperature of a gas. What is the manipulated charless law formula to find v2. Charles Law is expressed as.
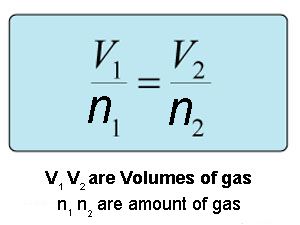
It is well known fact that volume of the given gas at constant temperature and pressure is directly proportional to the number of moles Avogadros law Vα n. When the pressure on a sample of a dry gas is held constant the Kelvin temperature and the volume will be in direct proportion. The equation of Charless law is V kT.
Which best explains why the pencil seems bent. So by these equations. The Charles law mathematical expression will be.
The equation of Charless law is V kT. Similarly V₂ and T₂ are the final values of these gas parameters. Where V is the volume of the gas.
VT k where V is the volume of gas T is the temperature of gas measured in kelvins and k is a constant. For 1 degree rise in temperature volume increases V 0 1 273. Charles Law states that at constant pressure the volume of a fixed mass of a dry gas is directly proportional to its absolute temperature.
V_1 first volume V_2 second volume T_1 first temperature T_2 second temperature Send. What is the first name of the scientist who formulated charless law. Here we should remember that the temperatures are absolute temperatures that are measured in Kelvin not in ⁰F or ⁰C.
A modern statement of Charless law is. Derivation of Charles Law. The law was discovered by Jacques Charles in the late 1700s.
What is the formula for charles gas law. French physicist Charles studied the effect of temperature on the quantity of a gas at constant pressure. It states the volume of a gas is directly proportional to its temperature unless the pressure and the amount of the gas remain constant.
V₁ T₁ V₂ T₂ where V₁ and T₁ are initial volume and temperature respectively. V F Final volume. What is the mathematical formula for charles law.
Charles law is an experimental gas law. As per the definition of Charles Law we can write the equation as V₁ T₁ V₂ T₂ where V₁ and T₁ are the intial volume and temperatures and V₂ T₂ are the final values. According to this formula at a fixed pressure the volume of a gas is proportional to the temperature of the gas.
Vlada 557 6 months ago. If the temperature of the balloon is increased to 40 K what will the new volume of the balloon be. Mathematically Charles Law can be expressed as.
Temperature must be in complete units ie K At 290 degrees a sample of nitrogen gas has a volume of 390L. Charles law formula. What is the formula used in charles law.
As we can see from the above equation the law relates the volume of gas to its temperature. In the 1800s a French scientist named Jacques Charles made discoveries regarding the effect of temperature on gases. Charles Law There are 65 liters of helium in a balloon at 35 K.
You might be interested in. T F Final absolute temperature. The constant is R which is called general gas constant.
We can represent this using the following equation. As we can see from the above equation the law relates the volume of gas to its temperature. For t degree rise in temperature volume increases V 0 1 273 t.
V i T i V f T f where V i initial volume T i initial absolute temperature V f final volume T f final absolute temperature It is extremely important to remember the temperatures are absolute temperatures measured in Kelvin NOT C or F. DescriptionCharless law is an experimental gas law that describes how gases tend to expand when heated. T I Intial absolute temperature.
Let say you want to determine the final volume then the. What is the charles law formula What is the general formula of charles law. Charles law states that at a constant pressure the volume of a given mass of gas is directly proportional to the absolute temperature.
According to Boyles law. Based on the definition of Charles law we can write the Charles law equation in the following way. The formula created by Charles was frac V_1 T_1 frac V_2 T_2 We can also further consider this to give frac V_1 T_1 frac V_2 T_2 frac V_3 T_3 frac V_4 T_4.
It explains how gases tend to expand when heated. Let V 0 be the volume of a given mass of a gas at 0 C and V t is its volume at any temperature t C then the volume V t may be written in terms of Charles law at constant pressure as. Where V I Initial volume.
Equations like the one below are now used. The law was discovered by Jacques Charles in the late 1700s. Charles law formula V₁ T₁ V₂ T₂ where V₁ and T₁ are initial volume and temperature respectively.
What is the formula for Charles Law. Charles Law Formula. Charles Law Formula.
EqV propto T eq Where eqV textVolume of the gas eq eqT textAbsolute Temperature of the Gas eq. Similarly V₂ and T₂ are the final values of these gas parameters. Similarly V₂ and T₂ are the final values of these gas parameters.
What is the Formula of Charles Law. Charles law states that When the pressure on a sample of a dry gas is held constant the Kelvin temperature and the volume will be in direct proportion. Charles Law formula is written as V I T I V F T F.
According to Charless law. During this article well discuss Charles law formula its properties etc. According to Charles law equation.
AnswerIt states that the volume of a fixed mass of a gas is directly proportional to the temperature.

Boiles Law And Charles Law Boyle S Law Charles Law Hyperbaric Oxygen Therapy

Charles Law Volume Temperature Lab Answers Charles Law Physics Laws Thermodynamics

Charles Law Charles Law Easy Science Chemistry Classroom

Gas Laws Definition Facts Formulas Examples Chemistry Ideal Gas Law Chemistry Lessons Gas Laws Chemistry

Ideal Gas Law Derivation Pv Nrt Ideal Gas Law Physics Facts Boyle S Law

Combined Gas Law Definition Formula Examples Ideal Gas Law Boyle S Law Physical Chemistry

Gas Laws Gas Laws Chemistry Chemistry Basics Study Chemistry

Charles S Law Definition Formula Examples Charles Law Teaching Place Values Law

Pin By Chemistry With Saliha On Physical Chemistry Charles Law Physical Chemistry Chemistry

Boyle S Law Youtube Boyle S Law Charles Law Hyperbaric Oxygen Therapy







Comments
Post a Comment